Toward Distant Suns:
Chapter 2 – The Terrestrial Hang-up Chapter 2 – The Terrestrial Hang-up
Toward Distant Suns
by T. A. Heppenheimer
Copyright 1979, 2007 by T. A. Heppenheimer, reproduced with permission
Chapter 2: The Terrestrial Hang-up
In thinking about the origins of life, it is hard to avoid the temptation to
add the caveat, “as we know it.” On Earth, living organisms are built from compounds of carbon and nitrogen and live with the aid of oxygen. As Carl Sagan has aptly noted, some scientists claim that life must inevitably be so, but these scientists are biased since they themselves are built from compounds of carbon and nitrogen and breathe oxygen. Is this bias reasonable?
One alternative is that life might be based not on carbon but on silicon, which is chemically quite similar and in addition is more abundant. A great advantage of carbon for life is that when it bonds with other atoms to form compounds, the bonds are relatively weak and easily broken. Thus such compounds can partake in very subtle reactions. Also, carbon forms an incredible variety of compounds and combines readily with such key elements as oxygen, hydrogen, phosphorus, nitrogen, and sulfur. Silicon, by contrast, forms very tight chemical bonds; rocks are compounds of silicon, which is why they are hard and not susceptible to change. Still, a variant group of compounds known as silicones indeed are very similar to some carbon compounds. Could silicones serve as a basis for life?
To answer this, we must think of the first origins of life. Life began amid mixtures of simple compounds: ammonia, methane, water, hydrogen cyanide, carbon dioxide, phosphates. These were stirred and combined by the action of various natural energy sources, eventually forming more complex compounds: amino acids, the precursors of proteins; nucleic acids, the incipient genetic code; simple sugars; fatty acids; and the like. Charles Darwin had the basic idea as early as 1871, when he wrote: “If (and oh! what a big if!) we could conceive in some warm little pond, with all sorts of ammonia and phosphoric salts, light, heat, electricity, etc., present, that a proteine compound was chemically formed ready to undergo still more complex changes, at the present day such matter would be instantly devoured.”
How might we introduce silicon into such reactions? Carbon dioxide is CO2; the analogous silicon compound is Si02. But this is simply sand, a very stable compound not readily incorporated into chemical reactions. We begin to get somewhere by thinking of methane, CH4; its silicon counterpart is silane, SiH4. This compound is not at all so tightly bound and might well take part in biochemistry. The trouble is, it is like the Wicked Witch of the West in The Wizard of Oz. It is very sensitive to water. Even small traces of water will break it apart, and water is very, very abundant. Water (or steam) then would act to poison the prospects for silicon-based life by blocking at the start the pathways by which it could arise. (This does not rule out the possibility that silicon-based life may arise out of carbon-based life, but this idea will be deferred to another chapter.)
Somewhat more sanguine hopes may exist for another often-suggested possibility: life based not on water but on ammonia. Ammonia in many ways behaves similarly to water and is liquid over the range of temperatures of -108° to -28° F. This is rather narrower than the range for water, 32° to 212° F, but this need not prove to be a handicap. There are many small, cool stars around which worlds might orbit, with low temperatures suitable for lakes or oceans of ammonia.
The biochemist P.M. Molton has carefully examined how ammonia could substitute for water in the chemistry of life. This substitution would have to cover a number of important processes: action as a solvent; maintenance of a chemical balance within cells; formation of compounds analogous to proteins, fatty acids, nucleic acids, carbohydrates, lipids, steroids, phosphate compounds; production of energy, by processes akin to respiration. In a beautiful and detailed paper, Molton showed in 1974 how ammonia could do all those things and more. He even found ammonia-based analogues to the main energy-producing reactions in cells, the so-called Krebs cycle. In the Krebs cycle, citric acid serves to speed the breakup of glucose (a form of sugar), which is combined with oxygen to yield energy. If ammonia-based creatures exist, they would not breathe oxygen and exhale CO2 . Instead, they might well breathe (or drink) ammonia, and excrete a substance known as cyanamide, NH2CN.
However, there is a problem in this. Such a planet would be bathed in ultraviolet radiation from its parent star, which would penetrate through the atmosphere to ground level as well as many feet deep into the oceans of ammonia. The radiation would pose a serious danger to life since it breaks proteins apart. On Earth we are shielded from this by a layer of ozone high in the stratosphere; the small amount of ultraviolet that leaks through nevertheless is quite sufficient to burn the skin, as a visit to the beach on a sunny day will quickly prove. In the absence of ozone, a form of oxygen, small living creatures could not survive on land. In fact, an ammonia-covered world would absorb oxygen, preventing its buildup in the atmosphere. There is no reason to think that ammonia-based proteins would be any less susceptible to damage from this radiation than are Earth’s water-based proteins. Of course, we can speculate about the ammonia creatures sheathing themselves in shells resembling the ultraviolet-blocking windows of airliners; but on the basis of Earth’s geologic record, we find this does not happen. Until our ozone layer formed, some half a billion years ago, Earth’s life was effectively restricted to simple, unintelligent forms living in the sea.
Since we are interested in the origin of beings ultimately capable of colonizing the Galaxy, it is indeed quite reasonable to restrict attention to life as we know it, based upon carbon and water. Then, how did we come to be? And how readily might the same evolution have occurred elsewhere?
It is a commonplace idea that Earth had to form at the right distance from the Sun, or else it would have been too hot or too cold for life. This is a much more complex matter than was realized even a few years ago, because the Sun has not always been at the same brightness that we see today. It has grown brighter over geologic time, emitting more energy, while Earth has stayed at the same average distance from it.
This brightening of the Sun is a very well-supported finding in astrophysics. It results from the buildup of helium in the Sun’s core, produced by fusion of its hydrogen. The exact amount of this brightening is somewhat uncertain, but the Sun today is probably about 35 percent brighter than it was 4.5 billion years ago, when Earth formed. (Some investigators have found values as high as 50 percent.) This means that if Earth existed then as it does today, it would have been colder, since the Sun was dimmer—so much that the oceans would have frozen solid. The subsequent slow brightening of the Sun would not have melted these oceans, since an ice-covered Earth would reflect most of the sunlight back into space. On such a world, life as we know it could not arise.
Evidently Earth escaped this fate. But how? The answer is that Earth’s primordial atmosphere was very different from what it is today. It functioned as a blanket that trapped heat, so that primitive Earth was warm enough to keep the oceans from freezing. This blanketing is known as the “greenhouse effect,” because Earth’s primitive atmosphere acted like the glass windows in a greenhouse, which allow sunlight to enter freely yet trap the resulting heat. Even today the greenhouse effect warms Earth’s surface by some 60° F. Several billion years ago this added warming may have amounted to over 170° due to the presence of ammonia and methane, which produce a large greenhouse.
So in order for Earth to avoid being too cold for life as the Sun was warming through geologic time, at all times there had to be enough greenhouse to keep the oceans from freezing. According to recent studies, this means maintaining an average temperature of at least 41° over the entire planet.
Why would a smaller average temperature freeze the oceans? The reason is that since this is an average, the polar regions would be much cooler. They would form massive ice caps, reflecting back more sunlight, reducing further the polar temperatures and causing the ice caps to grow. As this process continued, glaciers would creep toward the equator, and the polar ice regions would spread as the process fed on itself. Whether the glaciers would actually reach the equator would depend on Earth’s initial average temperature, since obviously the glaciers would slow their advance and tend to come into a balance with unfrozen, warmer equatorial regions. But below this initial average of 41°, there would be no such balance. This runaway glaciation would freeze the entire planet.
There also is the matter of Earth being too hot for life. This does not mean being close to a hellishly hot sun, which beats down with an unforgiving intensity that would boil water. Instead, it is a matter of having too much of that good thing, the greenhouse effect. There is such a thing as a runaway greenhouse, and that is what happened to Venus.
Venus today has temperatures of 800° to 900°, but early in its history it may have been as pleasant and clement as Earth. Initially, Venus’s atmosphere contained little or no water vapor or carbon dioxide, but active volcanoes soon released these gases in copious amounts. Both these gases trap heat effectively and have a substantial greenhouse effect; thus their release caused Venus’s temperature to rise. As more of these gases accumulated, the Venus greenhouse grew hotter, and water was never able to condense to form oceans. The importance of oceans is that they dissolve the CO2, which then can combine with calcium to form limestone. Earth’s massive deposits of limestone contain the CO2 that on Venus produced its thick, dense, smotheringly hot atmosphere. This was Venus’s runaway greenhouse: a rise in surface temperature due to buildup of CO2 in the atmosphere, which was not counterbalanced by other effects.
Someday Earth will suffer a similar fate. In aeons to come the Sun will continue to evolve and grow brighter, and there will be a warming of our oceans. With this, some of Earth’s limestone will dissolve, releasing more CO2 into the atmosphere, and more water will evaporate. The added CO, and water vapor will enhance Earth’s greenhouse, producing still more temperature rise, more CO2, more water vapor. All the while, Earth will be radiating heat back to space, and will tend to strike another balance, preventing temperatures from getting still hotter. This balance exists today, limiting Earth’s greenhouse to the 60° rise mentioned, but the balance will shift to higher and higher temperatures as the sun slowly brightens. Eventually the balance point will be hot enough to allow the equatorial oceans to boil. With this, all hope will vanish; in a short time all Earth’s water, and much CO2 from limestone, will enter the atmosphere. An interplanetary explorer will find Earth and Venus all but identical.
This digression into planetary science, this discussion of the runaway greenhouse and of runaway glaciation, sets the stage for a short history of Planet Earth. We may think of our planet as a comforting mother, but her history in fact was much more like the Perils of Pauline. [Author’s footnote: The computations for this history were made by Michael H. Hart of the Laboratory for Planetary Atmospheres, NASA-Goddard Space Flight Center.]
Four and a half billion years ago the newly formed Earth was a ball of rock and iron, laced with radioactive potassium and uranium, whose radiant decay heated the outer layers of Earth, producing volcanoes on a scale that can only be called Promethean. Amid tempestuous rumblings and along massive fissures or fault lines the whole world over, there were cone-shaped forms of young erupting volcanoes. Now smoking, now roaring, now merely glowing balefully with lava in the throat, time and again they expelled the red-hot magma, or exploded in sheets of fire. As the molten rock poured forth, great clouds of gas issued from vents and shrouded the young planet in mist. The volcanic gases were nearly all water vapor and CO2, but about 1 percent of their content was methane, and a somewhat smaller fraction was ammonia. Despite their small quantities, these last two gases provided most of the early greenhouse, so that Earth would not freeze in the feeble warmth of a younger sun.
The water vapor gathered in vast extents of clouds, then condensed in torrents of rain, which gathered to form the incipient oceans. Part of the CO2 entered the atmosphere, but more and more of it formed limestone as the oceans grew. Much of the ammonia broke down to form our atmosphere’s first nitrogen, but some remained. With the growing content of methane, these gases were Earth’s first atmosphere.
Never again would conditions be so favorable to the origin of life. Beneath the atmospheric greenhouse, mild, equable temperatures prevailed. The early shallow seas were stirred by volcanic eruptions and bombarded with solar ultraviolet radiation, lightning strokes, and the shock waves of impacting meteorites. These created chemical reactions in the atmospheric gases, producing organic chemicals of increasing complexity. In this, the ammonia and methane did more than produce a greenhouse, more than provide raw materials for the reactions of life. They prevented oxygen from destroying the delicate biochemicals. High in the atmosphere, solar radiation was breaking water vapor molecules and releasing oxygen, a deadly poison that would have destroyed the primitive carbon compounds evolving toward life. But as soon as any oxygen formed, it immediately combined with the ammonia or methane and was removed.
The precious biochemicals formed, polymerized, isolated themselves in droplets, and slowly groped toward life. This was chemical evolution, or survival of the fittest compounds. It was not yet the true evolution of Darwin, but it was to the degree that chemical structures formed with more and more of life’s attributes and tended to persist while others were destroyed and broken back to simpler chemicals. Within a few hundred million years of Earth’s origin, true life appeared, able to consume food and grow and to reproduce with a genetic code.
This early life was in the form of one-celled creatures resembling bacteria or algae. They had an extremely simple structure and were very small in comparison to present-day cells; yet how promising they were as they drifted to and fro, warmed by sunlight in the estuaries of ancient seas. They lived by consuming the complex biochemicals that had not evolved as far as life. By simple chemical steps resembling fermentation, these cells could break down their foods’ structures to release a modicum of energy. These processes were wasteful, inefficient, and utterly dependent on nature to synthesize more of these biochemicals.
The challenge was for these cells to develop better means of getting energy. The evolving bacteria responded by developing enzymes and organic dyes, which could obtain energy from the iron and sulfur compounds dissolved in the sea. With further evolution, there arose blue-green dyes related to chlorophyll. In some long-forgotten lake, the first verdant traces of cyanobacteria or blue-green algae appeared. Henceforth there would be plant life to grace Earth’s waters.
For all this, the prospects for life still were tenuous. As volcanoes continued their eruptions, the content of atmospheric methane and ammonia grew greater, its greenhouse stronger. Lacking this 36 effect, Earth’s average temperature would have been -65° or worse; with it, the average was 106° four billion years ago, and heading higher. There now was certainly no danger of runaway glaciation; quite the contrary. The danger now was from the runaway greenhouse. If the average were to reach 126°, this would become unavoidable, and life would perish in steam.
As Earth approached its billionth birthday, the volcanoes began to decline in power and the rise in average temperature became less steep. Yet the rise continued to 110° and even higher. Now, however, the growing presence of life began to make itself felt. As the algae spread, they carried out photosynthesis and produced oxygen. The oxygen combined with the ammonia and methane to remove some of these gases, and the rise in their content slowed as the work of the algae approached and then matched the work of the volcanoes.
For a hundred million years matters hung in the balance. Here were the volcanoes, diminishing in action yet still powerful, every eruption a reminder of Earth’s peril. There were the algae, growing in thin mats, freshening the waters with the oxygen of hope. Here were globe-embracing thicknesses of cloud, fed by the plutonic fires, dimming the weak sunlight and bidding to make Earth permanently a place of fire and darkness, of stifling, choking, never-breaking overcast. There the algae were accepting the feeble light, growing and budding in their turn, taking up the challenge: Would life predominate, and remake Earth after its own nature? Or would fire and steam be the victors, and thereafter rule the world?
At last the issue came to a resolution, and we are evidence that the decision was on the side of life. The content of ammonia and methane peaked, then began to decline as the algae made their presence felt with increasing strength. The greenhouse also peaked, with Earth ‘s average temperature topping out at 111° or 112° F. Thereafter, temperatures began to decline. Earth had passed its first crisis in the evolution of life—and it was the humble blue-green algae that saved the world for us all.
The next billion or so years were increasingly peaceful. It was the time of simple one-celled life, now developed into a community of plants and animals; that is, of algae, which could photosyn¬thesize, and of bacteria, which could engulf and consume the primitive plants. This was the first food chain, but life was still crude, primitive. Nor was it widespread. Since there was still no free oxygen in the sea or atmosphere, the cells lived by the inefficient process of fermentation. Most of the energy in food went to waste, for want of oxygen with which to extract it.
Life was limited in extent for another reason. Earth was bathed in solar ultraviolet, which rendered the land unfit for life and which penetrated deep into the oceans. No life could exist in the surface layers; yet algae could not grow too deep, for they required the pale sunlight that filtered through to depths where the ultraviolet could not reach. At greater depths the sunlight itself could not penetrate. In this thin layer of water, between the extremes of too much ultraviolet and too little sunshine, life found its milieu.
Slowly, slowly the levels of methane and ammonia fell while those of nitrogen rose. With this, temperatures fell too: to 88° three billion years ago, to 75° a half-billion years later. By now the Sun was beginning to evolve and its core showed the first traces of helium. Its temperature rose imperceptibly, as did its brightness. Some two billion years ago, the oxygen from photosynthesis succeeded in removing the last traces of ammonia and methane; Earth’s atmosphere then was nearly all of nitrogen, with a few percent of water vapor and carbon dioxide. Now a new problem arose. With these last traces gone, their greenhouse was gone too, and in the short time of a quarter billion years, temperatures plunged over twenty degrees. Now the danger of runaway glaciation became real, as the global temperature dropped into the forties. With nothing left but CO, and water vapor to give the atmosphere its greenhouse, this global mean fell to 46° two billion years ago; then 45°, 44°, 43°. The margin against disaster was only two degrees.
The ancient Norse saga, the Eddas, tells of the legendary Fimbul-winter, when the world was locked in ice and snow, with fierce and bitter winds howling from the dark and brooding cloudbanks to the north. Not for a mere hundred million years but for a billion, this was the world north and south of the tropics. Here was the majesty and splendor of the arctic, embracing far more than today’s mere polar patches. Here was the Sun sparkling on the crystals of new-fallen fields of snow on rare days when the sky turned a brilliant blue. Then again there were the endless, trackless stretches of pack-ice, of jagged blocky floes extending past the horizon, past many horizons. There were glaciers, extending perhaps to lands where corals grow today, breaking at their edges to release massive bergs in sprays of splashing water and rime-ice. And always, always, the bitter whistling winds, in air thinner than
today’s, their moanings and piercing calls ceaselessly repeating the message that those who live must die.
No life could survive in the lands of ice, but in the equatorial regions it made its stand. It could not now act to save itself, to alter its fate by growing and spreading, or by releasing oxygen. Still, the ocean and atmosphere were in equilibrium and there would be no reduction in the atmospheric CO, or water vapor to eliminate the slim margin of greenhouse effect that held back the glaciers. In the end, temperatures bottomed out just below 43° and slowly rose, degree by degree, as the billion years elapsed. It was not life that did this, or any other process of Earth. It was the Sun, slowly gaining its strength and brightness. As the aeons passed, the global mean temperature rose into the high 40s, then 50s. The danger of a world of ice was even more real than the early danger of a world of steam; but it too passed.
The low temperatures at the minimum had not threatened life, but as algae produced more oxygen, there came a time when that gas could begin to build up in the atmosphere. When the last ammonia and methane were gone, free oxygen appeared for the first time, in the amount of some 5 percent of the present level. We would find this quite inadequate; we would suffocate in such a world. Yet even this modest level of oxygen wrought a revolution in the history of life.
Instead of using fermentation, it now became possible for cells to obtain energy by respiration, 40 by combining sugars and other foodstuffs with this oxygen, bringing an immense gain in the energy available to life. With fermentation, in the absence of oxygen, a molecule of glucose produced two units of energy; that is, two phosphate bonds in the substance known as adenosine triphosphate or ATP, which is the universal medium of exchange of living energy. With respiration, a molecule of glucose yielded not two but thirty-six such phosphate bonds. The consequence was somewhat as if a car’s gasoline mileage were to improve eighteen-fold.
This change stimulated an increase in the complexity of life. The earlier cells had been small, with little or no cellular structure or internal parts. They were merely blobs of living matter enclosed within simple cell walls with their genetic material, DNA (deoxyribonucleic acid), as a loop within. The more complex cells which evolved were much larger, and came to have many of the features of cells in today’s plants and animals. Their chlorophyll was bound up in specialized centers or chloroplasts. Other cellular structures, the mitochondria, arose to handle the digestion of food by respiration. Some of these cells developed efficient means of moving about with whiplike appendages known as flagellae. Most importantly, the DNA came to be organized into chromosomes located within cell nuclei. The evolution of nuclei and chromosomes brought modern forms of cell division and opened the way for the advent of sexual reproduction, which relied on these modern forms. This advance was a vast improvement over the primitive buddings and splittings of bacteria that had existed before.
The increase in cell complexity may seem minor, yet it was a greater step for life than the origin of plants and animals. As long as there were only the bacteriumlike forms of the days before free oxygen, there could be nothing more than one-celled life. The new, more complex cells could organize into tissues; life could come to be of many cells. That would not come about immediately, but even a billion and a half years ago, the revolution of more-complex cells had brought to these basic units of life the structure and functions that they have today. [Author’s footnote: What here are described as “bacteriumlike cells” and “complex cells” are known to the biochemist as prokaryotes and eukaryotes. All the cells in today’s plants and animals are eukaryotic.]
The initial buildup of atmospheric oxygen did not proceed very far despite the great impetus it gave to life. The solar ultraviolet still restricted life to regions beneath the sea; in addition, the oxygen soon found other chemicals with which to combine. Two billion years ago, the seas were full of dissolved iron. The initial free oxygen in seawater acted on this iron, and the seas slowly rusted. The dissolved iron changed from a soluble form to an insoluble one, and like snowflakes falling from the air, particles of iron oxide gently formed and fell to the seabed. These in time would be the world’s economic reserves of iron ore, but that was far in the future. Even more oxygen was used up in reactions involving sulfur.
For a billion and a half years, oxygen levels in the atmosphere rose slowly, from 5 percent of present-day values two billion years ago to 8 percent. For much of this time life was still limited to the narrow region of water between the zones of too much ultraviolet and too little sunlight—between the devil and the deep blue sea, as it were. Nearly as fast as oxygen was produced, it combined with iron or sulfur. Still, life was not without its advantages. High in the stratosphere, sunlight transformed some oxygen into ozone, which shielded out some of the ultraviolet. This meant green plants could grow closer to the surface, and life could not only occupy a greater range of ocean depths but also could take advantage of the more intense sunlight in the shallows of oceans.
About half a billion years ago, with oxygen at 8 percent of the present level, the ozone layer grew thick enough to permit plants to grow in open air, without any protection by a depth of water. They could then grow on the very surface of the sea, taking full advantage of sunlight and releasing oxygen directly into the atmosphere. The protection of ozone meant that the first plants could creep landward from the shallows and colonize what heretofore had been bare rock. The result was a vast flowering or proliferation of life and a rapid rise in oxygen levels. A hundred million years saw the oxygen level quadruple to one-third the present level. By three hundred million years ago, the level had passed half the present value and was still rising.
This increase sparked a remarkable diversification of life forms. In the short time of the geologic epoch known as the Cambrian, when oxygen levels were quadrupling, virtually all the principal types of Earth’s life forms appeared, including not only the major forms of invertebrates but also primitive creatures possessing a notochord, a structure which in modern embryos develops into vertebrae. This Cambrian revolution saw the advent of animals with bones or shells, which left permanent fossil impressions. The Cambrian epoch was followed by others but the pattern of life’s development was clear: increasing complexity, increasing diversity. A few more hundreds of millions of years of Earth’s history, and one of these complex forms would gain the wit to look back and contemplate these changes, and to wonder.
And among the things we may wonder about is: How much of this are we to believe?
There is good geological evidence for the first appearance of free oxygen some 2 billion years ago, for the first complex cells 1.5 billion years ago, and of course for the Cambrian epoch. Evidence for the early times of heat and cold is much more scanty and ambiguous. There is good evidence for the existence of liquid water some 3.2 billion years ago, but it does not help determine its temperature. Tillites, a form of rock associated with glaciers, have been found from 2 billion years ago, which would be expected if that was a time of great cold. Yet other scientists argue that Earth’s climate then was even warmer than it is today. So it must be emphasized that the history of Earth given here has been derived from calculations and only partly from the geologic record.
However, a number of different scientists have studied the question of Earth’s fate if it had formed slightly closer to the Sun, or slightly farther. These studies are much more pertinent to the true concerns of this chapter and far less dependent on the geologic record. With minor variations, they agree with the conclusions to be drawn from the work of Michael Hart discussed here: Earth would have suffered a runaway greenhouse if it had formed more than 5 percent closer to the Sun. It would have frozen had it formed more than 1 percent or 2 percent farther out.
Thus did our Earth evolve, and the life upon it, according to these ideas. Many scientists have long argued that life would arise whenever conditions were favorable, but this does not necessarily mean the life would be more than the simplest bacteria or algae. There would have to be two other events before life could advance to offer the prospects of future intellect. First, there would be need for an initial quantity of oxygen free in the atmosphere to bring the revolution of increased cell complexity, producing cells as we know them today. Second, there would be need for increased oxygen levels to promote formation of the ozone layer and permit life to emerge from beneath the water. This event would lead to the Cambrian revolution, with its vast increases in plant and animal complexity. [Author’s footnote: These ideas were first put forth in a slightly different form by L.V. Berkner and L.C. Marshall in the mid-1960s.]
On Earth, these three revolutions—initial origin of life, origin of complex cells, and the Cambrian revolution—all came essentially to completion within a few hundred million years of their onset. Since this time is short compared to the billions of years of Earth’s history, we are justified in saying that on other planets, similar atmospheric changes would soon bring about similar advances in life’s evolution.
All these developments depended quite critically on three things, fulfillment of which was a matter of chance. The Sun had to be the right mass. The Earth had to be the right mass. And the Earth had to be the right distance from the Sun.
The last of these is the simplest to understand. Had Earth formed at less than 95 percent its present distance, the early greenhouse would have run away and our oceans would have boiled. From that day forward, Earth would have been a lifeless planet resembling Venus. Had Earth formed beyond 101 percent its present distance (that is, more than 1 percent farther out), it would have frozen over some two billion years ago due to runaway glaciation, in Hart’s calculations, and today would resemble Mars.
What of the size of Earth? If it had formed a bit farther out so as to just avoid (by an even narrower margin) freezing over when the primordial ammonia and methane were removed, it could be no more than 30 percent more massive than it actually is. That is, its diameter could not exceed 8,639 miles, compared to the actual 7,926. A larger Earth would have had more internal radioactivity and hence would have formed its early atmosphere more rapidly, so much so that the early buildup of ammonia and methane, prior to the advent of photosynthesis in algae, would have taken place more quickly and forced a runaway greenhouse.
By contrast, if Earth had formed a bit farther inward so as to have avoided this initial runaway by a very narrow margin, it still would have had to avoid runaway glaciation when the algae removed the ammonia and methane. It thus could be no less than 14 percent less massive than it is, or with a diameter less than 7,530 miles. Otherwise, atmospheric evolution would have progressed more slowly; and when Earth faced the crisis of runaway glaciation, there would have been insufficient CO, and water vapor in the atmosphere to yield a greenhouse sufficient to avert this fate.
The matter of the Sun’s mass also deserves note. If the Sun had formed with more than 10 percent additional mass, it would have begun emitting considerable amounts of ultraviolet radiation by the time it was four billion years old. This occurrence would have inhibited the Cambrian revolution. To combat this radiation, Earth would have had to have formed farther from the Sun. The presence of this excess radiation thus would have cut into the range of distances over which Earth could have formed so as to progress to the Cambrian revolution. If the Sun had formed with more than 20 percent additional mass, its evolution would have progressed so rapidly that by the age of four billion years its temperature would have increased very quickly. This would have driven Earth to a runaway greenhouse at virtually any reasonable distance.
A much smaller sun would have posed a different problem. Small stars evolve less quickly; the growth of their brightness with time is slower, less marked. Let us remember that 2 billion years ago, when Earth had escaped the early threat of a runaway greenhouse only to face runaway glaciation, what saved our world was that the Sun had increased slightly in brightness over the previous 2.5 billion years. With less than 83 percent of its present mass, the Sun would have evolved so slowly as to have failed to have offered those critical few degrees of extra temperature when needed. The removal of the last traces of ammonia and methane then would have brought runaway glaciation. [Author’s footnote: In these calculations. Michael Hart assumed that Earth could have formed at any distance from the Sun, so as best to improve its prospects for life.]
The concept of Earth’s history as being poised so delicately between fire and ice is reminiscent of Dr. Pangloss in Voltaire’s Candide, who opined that everything is for the best in this best of all possible worlds. Such a conclusion may seem strange, particularly at income tax time; yet it was Robert Frost in his poem, “It Bids Pretty Fair,” who put things in perspective:
The play seems out for an almost indefinite run.
Don’t mind a little thing like the actors fighting.
The only thing I worry about is the Sun.
We’ll be all right if nothing goes wrong with the lighting.
This then is the terrestrial hang-up: that to form a world suitable for life as we know it, there must be what actually is a very good replication, obtained purely by chance, of our system of Sun and Earth. So if ours is indeed the best of all possible worlds, more or less, then we should be able to estimate how many similar worlds may be found. This can be done by using the ideas of the planetary hang-up and the terrestrial hang-up.
To begin, the age of the Galaxy is known: some fourteen billion years. Throughout most, if not all of that time, stars have been forming, some twenty per year. However, not all of them would be of interest in a search for extrasolar civilizations. The first-formed stars were almost entirely of hydrogen and helium. As they evolved, they built up heavy elements such as carbon, oxygen, silicon, nitrogen-elements from which planets could form. The early history of element-building in the Galaxy is somewhat unclear, but from what we know of astrophysics it seems reasonable that for stars formed in the first three billion or so years, there were insufficient heavy elements to permit planets to form.
Also, stars that have formed relatively recently are too young for us to expect intelligent life to have arisen. Earth is a most flourishing habitat; there can be few planets on which life has developed more exuberantly. Yet it has taken all but the last few million years of earth’s history to develop what we call, parochially, “intelligence.” If our history is representative, then stars formed more recently than the Sun are not of interest. The age of the Sun is known: 4.5 billion years.
So we are interested in the stars formed between 4.5 and 11 billion years ago; these number some 130 billion. Not all of these are potential abodes for planets. Some are hot, bright, blue stars like Altair and Sirius. These burn out so quickly, in no more than a couple billion years, that their worlds will perish long before life can well develop. Many more stars are small and cool, the so-called red dwarfs. These will burn for hundreds of billions of years, but like a candle in a window, they provide little heat at a distance. Hence their planets would be expected to be too cold for life. To be more specific, we can construct a table:
PROBABILITIES FOR EXISTENCE OF EARTHLIKE PLANETS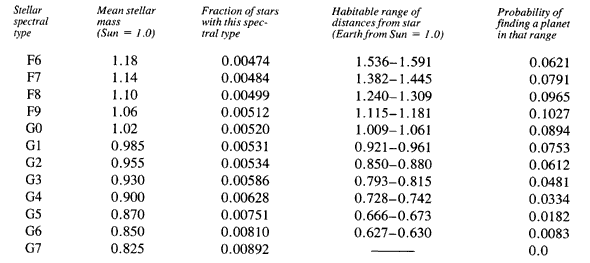
[Data in the first three columns are taken from Habitable Planets for Man by Stephen H. Dole, (New York: Blaisdell, 1964), pp. 102 and 104. The fourth column was computed from equations given by Michael Hart; see references to his papers in the Bibliography. The last column again uses data by Dole, pp. 91 and 92.]
A bit of explanation is in order. Astronomers classify stars by the appearance of their spectra, thus furnishing a convenient way to refer to groups of similar stars. The first column gives the astronomers’ code for this classification. The fourth column gives the range of planetary distances over which Earth might have formed so as to avoid thermal runaways while progressing to the Cambrian revolution. The last column assumes that planet distances are distributed as in our own solar system. For example, a typical star in spectral class F9 has a mass 6 percent greater than the Sun; some one-half percent of all stars are of this type. One would find an earthlike planet only between distances from that star of 1.115 and 1.181 times Earth’s distance from the Sun, but there is a bit better than a 10 percent chance that a given star of that type would actually have a planet there.
Of the 130 billion stars that are the right age, a great many of them suffered the planetary hang-up and failed to form worlds such as Earth. In view of the carnage wrought by Jupiter within our solar system, it is risky to imagine that planets exist where a star has a binary companion larger than Jupiter. Following the estimates of Abt and Levy, discussed in Chapter 1, this would rule out 90 percent of these stars. We thus have 13 billion candidates.
From the table, we find that only about one-third of 1 percent of these would have the right combination of stellar mass together with a planet in the proper range of distances. We thus are down to 46 million planets. Earlier we noted that to avoid the end of life, a planet must be between 86 percent and 130 percent the mass of Earth. If planets throughout the Galaxy have the same distribution of masses as in our own solar system, then there is only a chance of 1.9 percent that a given planet has the right mass. This brings us finally down to 880,000 worlds like ours in the Galaxy.
We have not considered the suggestion that binary companions resembling large versions of Jupiter might have systems of worlds in close orbit, like the Galilean satellites. All four of our Solar System’s large planets have such collections of large satellites, these being Jupiter, Saturn, Uranus, and Neptune. However, the mass of the largest Jupiter satellite, Callisto, is 0.0246 times that of Earth. Jupiter is 18.5 times more massive than Neptune, but the largest satellite of Neptune (Triton) is 0.0227 times Earth’s mass or 92 percent that of Callisto. By coincidence, the mass of Triton is also very close to that of Titan, the largest satellite of Saturn. There thus is no reason to believe that even if a binary companion were much larger than Jupiter, it could possess a group of close-circling satellites one of which were Earth-size. The processes that produce such worlds seem to limit their sizes to roughly that of the Moon.
So we finally come down to an estimate of 880,000 planets suitable for life. Nevertheless, this now is the real McCoy, the genuine nitty-gritty. We expect that each of them will be virtually a dead ringer for Earth, of similar size and appearance, with a similar star for its sun, and at the right distance and age to have experienced the Cambrian revolution upward of half a billion years ago. Moreover, it is likely that every one of them is (or soon will be) the abode of an intelligent species. These are just the worlds we would seek in space. Yet by these calculations, there is only one such planet for every 227,000 stars.
Our world is as a speck of dust lost in vastness, but to describe our sun as an ordinary or average star, as in the astronomy texts, is not correct. It is a very special star; its uniqueness lies in its being one of perhaps only 880,000 that are abodes for advanced forms of life. So it is not difficult to imagine that the other barren stars are calling to us, inviting us to cross the vast gulfs of distance, to find new lands, to make them fruitful.
This is the dream of space travel. This is also the dream of space colonization. We may realize such dreams in time, if the call of the distant stars is not to be denied. Should we one day reach out in this way, our efforts will be seen as flowing from today’s space projects, today’s space hopes. It will be a task for the future’s historians to trace the lineage from the space shuttle to the starship.
If this lineage is today unclear, still we may hope. It is with this hope that we may carefully examine our current space activities, with an eye to finding within them the germs of future advances. In this spirit, we may look carefully at the prospects for space flight along the way to the stars.
The stars are far, but close at hand is the space between Earth and the Moon. It is here that we may build. The possibilities in this near-space must necessarily concern us, and must be explored in detail, before we can speak again of the stars. These possibilities are the subject of the next several chapters, and the prospects to be kept in mind are that they will include, as an activity beneath the orb of the Moon, the colonization of space.







